More info at Senoinflammation or Inflammaging or …??? | Biopsychosocial Medicine and InflammAging in general and local .. | Quantum Medicine (and sub page)
InflammAging (Claudio Franceschi, 2000) has, tanks to recent knowledge development, become importantly be considered: ”Inflamm-aging (also known as inflammaging or inflamm-ageing) is a chronic, sterile low-grade inflammation that develops with advanced age, in the absence of overt infection, and may contribute to clinical manifestations of other age related pathologies. Inflammaging is thought to be caused by a loss of control over systemic inflammation resulting in chronic, overstimulation of the innate immune system. Inflammaging is a significant risk factor in mortality and morbidity in aged individuals [2] ..
Current research studying inflammaging is focused on understanding the interaction of dynamic molecular pathways underlying both aging and inflammation and how they change with chronological age.” https://en.wikipedia.org/wiki/Inflammaging
… “Cytokine dysregulation is believed to play a key role in the remodeling of the immune system at older age, with evidence pointing to an inability to fine-control systemic inflammation, which seems to be a marker of unsuccessful aging. This reshaping of cytokine expression pattern, with a progressive tendency toward a pro-inflammatory phenotype has been called “inflamm-aging.” Despite research there is no clear understanding about the causes of “inflamm-aging” that underpin most major age-related diseases, including atherosclerosis, diabetes, Alzheimer’s disease, rheumatoid arthritis, cancer, and aging itself. While inflammation is part of the normal repair response for healing, and essential in keeping us safe from bacterial and viral infections and noxious environmental agents, not all inflammation is good. When inflammation becomes prolonged and persists, it can become damaging and destructive. Several common molecular pathways have been identified that are associated with both aging and low-grade inflammation.” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5900450/
Inflammaging determines health and disease in lumbar discs-evidence from differing proteomic signatures of healthy, aging, and degenerating discs https://pubmed.ncbi.nlm.nih.gov/31125691/ “.. discs” https://pubmed.ncbi.nlm.nih.gov/31125691/ See more the link, e.g. ”The true understanding of aging and disc degeneration (DD) is still elusive. MRI has not helped our attempts to understand the health and disease status of the discs as it reflects mainly the end morphologic changes and not the changes at a molecular level.” … Conclusions: Our study documented diverse proteome signatures between the young, aging and degenerating discs. Inflammaging was the main differentiator between normal biological aging and DD (disc degeneration). Clinical significance: Multiple inflammatory molecules unique to DD were identified, allowing the possibility of developing specific biomarkers for early diagnosis and thereby provide evidence-based metrics for preventive measures rather than surgical intervention and also to monitor progress of the disease.”
Inflammaging: Age and Systemic, Cellular, and Nuclear Inflammatory Biology in Older Adults https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6777092/ “STAT signal transducer and activator of transcription … Within a community-dwelling sample of older adults, older age is associated with increases in STAT activation, along with increases of systemic inflammatory cytokines. In older adults, heterogeneity in age-related increases in inflammatory disease risk may be related to individual variability in inflammation”
Source of Chronic Inflammation in Aging
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5850851/
“Aging is a complex process that results from a combination of environmental, genetic, and epigenetic factors. A chronic pro-inflammatory status is a pervasive feature of aging. This chronic low-grade inflammation occurring in the absence of overt infection has been defined as “inflammaging” and represents a significant risk factor for morbidity and mortality in the elderly. The low-grade inflammation persists even after reversing pro-inflammatory stimuli such as LDL cholesterol and the renin–angiotensin system (RAS). Recently, several possible sources of chronic low-grade inflammation observed during aging and age-related diseases have been proposed. Cell senescence and dysregulation of innate immunity is one such mechanism by which persistent prolonged inflammation occurs even after the initial stimulus has been removed. Additionally, the coagulation factor that activates inflammatory signaling beyond its role in the coagulation system has been identified. This signal could be a new source of chronic inflammation and cell senescence. Here, we summarized the factors and cellular pathways/processes that are known to regulate low-grade persistent inflammation in aging and age-related disease.”
Conclusion: “Ideally, inflammation should subside immediately after elimination of the pathogen and insult to allow normal tissue to be rebuilt. However, low-grade persistent inflammation occurs in the majority of older people, leading to degeneration of several organs. There is strong evidence that the development of age-related multi-factorial conditions such as cancer, cardiovascular disease, Alzheimer’s disease, type II diabetes, frailty, sarcopenia, and osteoporosis is associated with low-grade elevations of circulating inflammatory mediators. Considering that aging is a complex process that results from a combination of environmental, genetic, and epigenetic factors, focusing future work on interventions addressing selectively destroying senescent cells, namely, “senolytic therapies” in the aging host rather than by treating symptoms of disease or attempting to block the effects of the multi-source of inflammaging, will offer improved therapeutic opportunities (49–52).
Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6146930/
“Most older individuals develop inflammageing, a condition characterized by elevated levels of blood inflammatory markers that carries high susceptibility to chronic morbidity, disability, frailty, and premature death. Potential mechanisms of inflammageing include genetic susceptibility, central obesity, increased gut permeability, changes to microbiota composition, cellular senescence, NLRP3 inflammasome activation, oxidative stress caused by dysfunctional mitochondria, immune cell dysregulation, and chronic infections. Inflammageing is a risk factor for cardiovascular diseases (CVDs), and clinical trials suggest that this association is causal. Inflammageing is also a risk factor for chronic kidney disease, diabetes mellitus, cancer, depression, dementia, and sarcopenia, but whether modulating inflammation beneficially affects the clinical course of non-CVD health problems is controversial. This uncertainty is an important issue to address because older patients with CVD are often affected by multimorbidity and frailty — which affect clinical manifestations, prognosis, and response to treatment — and are associated with inflammation by mechanisms similar to those in CVD. The hypothesis that inflammation affects CVD, multimorbidity, and frailty by inhibiting growth factors, increasing catabolism, and interfering with homeostatic signalling is supported by mechanistic studies but requires confirmation in humans. Whether early modulation of inflammageing prevents or delays the onset of cardiovascular frailty should be tested in clinical trials.”
The role of nutrition in inflammaging https://www.sciencedirect.com/science/article/pii/S1568163722000381
- Inflammaging, a low-grade, chronic, sterile inflammatory state occurs in the elderly.
- Inflammaging contributes to the pathogenesis of ageing and age-related diseases.
- Nutritional status is critical for maintaining proper immune system functionality.
- Diet has a direct influence on inflammation and can counteract inflammaging.
- Intake of whole grains, vegetables, fruits, nuts and fish can decrease inflammation.
“Conclusions: Dietary components may affect inflammation directly, counteracting the low grade age-related inflammation. In this regard, healthy diets, including the Mediterranean diet, are associated with lower concentrations of inflammatory mediators, like C-reactive protein (CRP) and Tumor Necrosis Factor-α (TNF-α), that are hallmarks of inflammaging. Among the components of a healthy diet, a higher intake of whole grains, vegetables and fruits, nuts and fish are all associated with lower inflammation. One area of promising research is the microbiome-ageing interaction. Indeed, dysbiosis plays a role in sub-optimal metabolism, immune function and brain function and contributes to the poor health and impaired well-being associated with ageing. Modulation of the gut microbiota has shown promising results in some disorders. Additionally, the discovery of several molecular pathways associated with ageing, and the characterization of the beneficial effects of calorie restriction (CR) in modulating metabolic pathways and preventing inflammation, should encourage research on CR mimetics, drugs able to promote lifespan and extend healthspan.”
Age-related cerebral small vessel disease and Inflammaging https://www.nature.com/articles/s41419-020-03137-x
“Circulating biomarkers of CSVD (cerebral small vessel diseases) inflammation were classified as markers of systemic inflammation and markers of vascular inflammation/endothelial dysfunction6. Besides, four core MRI features have been identified as imaging markers of CSVD, namely white matter hyperintensities (WMH), lacunae, cerebral microhemorrhage (CMB), and perivascular space enlargement (EPVS)56.” … The regional analysis showed blood markers of vascular inflammation are often associated with deep perforating arteriopathy (DPA), while blood markers of systemic inflammation appear to be associated with cerebral amyloid angiopathy (CAA). Here, we discuss recent findings in the pathophysiology of inflammaging and their effects on the development of age-related CSVD. Furthermore, we speculate the inflammaging as a potential target for future therapeutic interventions to delay or prevent the progression of the age-related CSVD.
Fig. 1: The deep interactions between aging, inflammaging, and age-related CSVD.
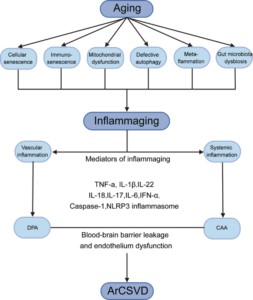
As aging, several cellular and molecular mechanisms lead to chronic inappropriate activation of the immune system. This complex interaction between genetic susceptibility and risk stimuli (both exogenous and endogenous) contributes to the continuous activation of a limited range of confounding sensors which triggers inflammaging (upper part of the box). The resulting synthesis and release of different inflammatory mediators are related to the common pathophysiological mechanisms of age-related diseases. For age-related CSVD, regional analyses showed that blood markers of vascular inflammation were associated with deep perforating arteriopathy (DPA), while blood markers of systemic inflammation were associated with cerebral amyloid angiopathy (CAA), both of which were closely related to the critical pathophysiological mechanisms of blood-brain barrier leakage and endothelial dysfunction (lower part of the box).”
InflammAging & Tinnitus
Neuroinflammation mediates noise-induced synaptic imbalance and tinnitus in rodent models https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.3000307 “…This excitatory-to-inhibitory synaptic imbalance was completely prevented by pharmacological blockade of TNF-α expression. These results implicate neuroinflammation as a therapeutic target for treating tinnitus and other hearing loss–related disorders.”
Chronic Inflammation – Inflammaging – in the Ageing Cochlea: A Novel Target for Future Presbycusis Therapy https://www.researchgate.net/publication/320354252_Chronic_Inflammation_-_Inflammaging_-_in_the_Ageing_Cochlea_A_Novel_Target_for_Future_Presbycusis_Therapy
Neuroinflammation and Tinnitus
https://pubmed.ncbi.nlm.nih.gov/34282564/
” Neuroinflammation is the central nervous system’s response to: injury, infection, and abnormal neural activity. Inflammatory processes are known to mediate many diseases, and recently evidence indicates that neuroinflammation underlies hearing disorders such as presbyacusis, middle-ear disease, ototoxicity, noise-induced hearing loss, and tinnitus. This chapter provides a review of the role of neuroinflammation in the etiology and treatment of tinnitus. Specifically, our research team has demonstrated that both tumor necrosis factor alpha (TNF-α) and calpain signaling pathways are involved in noise-induced tinnitus and that blocking them yielded therapeutic effects on tinnitus. Other efforts such as controlling acute inflammatory response via specialized pro-resolving mediators may help provide insight into preventing and treating tinnitus-related inflammatory processes”.
More of interests?
https://cmb.i-learn.unito.it/pluginfile.php/5044/mod_resource/content/1/Minciullo_et_al.pdf
https://www.mdpi.com/2073-4409/11/3/359/htm
https://rmdopen.bmj.com/content/rmdopen/7/1/e001470.full.pdf
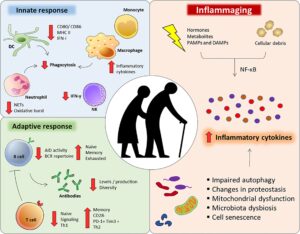
https://www.frontiersin.org/articles/10.3389/fimmu.2020.579220/full
& https://immunityageing.biomedcentral.com/articles/10.1186/s12979-020-00196-8
Perhaps not updated yet! -> http://biopsychosocialmedicine.com/projects/senior-health/senoinflammation-or-inflammaging-or/
Senoinflammation or Inflammaging or …???
The below concerns very complex knowledge development but also represent a very fast knowledge development, where we can extract some superior information which has been known for some time. Important is to extract such knowledge in a concrete useful way individuals can “tailor” based on reasonable understandable information. Autophagy is one of the cornerstones as well as mitochondria funtitonality.
Most of the below is from different references and finally some of my own conclusions
Inflammaging: A chronic pro-inflammatory status is a pervasive feature of ageing. This chronic low-grade inflammation occurring in the absence of overt infection has been defined as “inflammaging” and represents a significant risk factor for morbidity and mortality in the elderly. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5850851/
(Febr. 2018)
Redefining Chronic Inflammation in Aging and Age-Related Diseases: Proposal of the Senoinflammation Concept https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6457053/ (April 2019)
Age-associated chronic inflammation is characterised by unresolved and uncontrolled inflammation with multivariable low-grade, chronic and systemic responses that exacerbate the ageing process and age-related chronic diseases. Currently, there are two major hypotheses related to the involvement of chronic inflammation in the ageing process:
- Molecular inflammation of ageing and
- Inflammaging. However, neither of these hypotheses satisfactorily addresses age-related chronic inflammation, considering the recent advances that have been made in inflammation research.
- A more comprehensive view of age-related inflammation, that has a scope beyond the conventional view, is therefore required. In this review, we discuss newly emerging data on multi-phase inflammatory networks and proinflammatory pathwaysas they relate to ageing.We describe
(a) the age-related up regulation of nuclear factor (NF)-κB signalling, cytokines/chemokines,
(b) endoplasmic reticulum (ER) stress,
(c) inflammasome, and
(d) lipid accumulation.
The later sections of this review present our expanded view of age-related senescent inflammation, a process we term “senoinflammation”, that we propose here as a novel concept. As described in the discussion, senoinflammation provides a schema highlighting the important and ever-increasing roles of proinflammatory senescence-associated secretome, inflammasome, ER stress, TLRs, and microRNAs, which support the senoinflammation concept. It is hoped that this new concept of senoinflammation opens wider and deeper avenues for basic inflammation research and provides new insights into the anti-inflammatory therapeutic strategies targeting the multiple proinflammatory pathways and mediators and mediators that underlie the pathophysiological ageing process.
- Causes. As inflamm-aging (https://en.wikipedia.org/wiki/”Inflammaging) is a complex and systemic issue, it is likely that inflammaging is a result of several factors. It seems that the major cause of inflamm-aging is accumulation of misplaced and misfolded self-molecules from damaged cells”. …. “As inflamm-aging is a complex and systemic issue, it is likely that inflammaging is a result of several factors. It seems that the major cause of inflamm-aging is accumulation of misplaced and misfolded self-molecules from damaged cells.[10] These molecules are recognized by receptors of innate immune cells which leads to their activation and consequently to inflammation. Cell components which can stimulate innate cells include microRNAs, mitochondrial DNA or histones.
Senescent cells increase with aging, and senescent cells secrete a pro-inflammatory cocktail of chemicals, a condition known as senescence-associated secretory phenotype (SASP).[11] (from https://en.wikipedia.org/wiki/Senescence-associated_secretory_phenotype) SASP expression is induced by a number of transcription factors, including C/EBPβ, of which the most important is NF-κB.[6][7] NF-κB is expressed as a result of inhibition of autophagy-mediated degradation of the transcription factor GATA4 *.[8] GATA4 is activated by the DNA damage response factors, which induce cellular senescence.[8]Inflamm-aging has been also associated with persistent Cytomegalovirus infection. Cytomegalovirus drives up production of a variety of inflammatory cytokines and also results in expansion of CMV specific memory T cells.[12] Other possible factors that may lead to inflamm-aging include overnutrition, altered gut microbiome, impaired intestinal epithlial barrier, and chronic stress occurring in any stage of the individual’s life.[13][14][15] Cytokines with inflammatory properties can also be secreted by fat tissue” - Metformin Enhances Autophagy and Alleviates Inflammaging https://www.lifespan.io/news/metformin-enhances-autophagy-and-alleviates-inflammaging/
A recent study published in Cell Metabolism has shown that metformin, a drug that has been previously shown to be effective against some aspects of aging, ameliorates inflammaging by promoting autophagy, the cellular recycling of damaged components.Mitochondrial dysfunction, autophagy, and TH17Mitochondrial dysfunction is one of the hallmarks of aging, the root causes that cause us all to age. The mitochondria are the powerhouses of the cell, but as we age, our mitochondria have their DNA damaged by sources such as reactive oxygen species, harming their ability to perform their fundamental job. This gradual increase in dysfunctional mitochondria causes our cells to lose their ability to utilize energy, leading to a panoply of age-related diseases.Autophagy is the consumption of damaged or dysfunctional organelles by intracellular processes. Through autophagy, cells are able to recycle and renew their internal components, and, as the researchers of this paper explain, CD4+ (helper) T cells that do not properly perform autophagy behave like older cells.
Cytokines are known for causing inflammation, and, as the researchers demonstrate, the TH17 subset of cytokines is strongly associated with the chronic, age-related inflammation known as inflammaging, a key driver of multiple age-related diseases.
In order to demonstrate the relationship between autophagy, mitochondrial dysfunction, and TH17, the researchers used RNA silencing to disrupt the ability of cultured cells to engage in autophagy. Such cells that were taken from a young subject had their mitochondrial function decreased to the level of an older person. This RNA silencing also set the TH17 cytokine profile of these cells to become one associated with age and diabetes.
Obviously, what we want is the reverse of this process, and the researchers found that metformin, a commonly researched drug in the rejuvenation biotechnology field, was able to accomplish this in human cell cultures, spurring autophagy and reversing the TH17 cytokine profile.
Summary Age is a non-modifiable risk factor for the inflammation that underlies age-associated diseases; thus, anti-inflammaging drugs hold promise for increasing health span. Cytokine profiling and bioinformatic analyses showed that Th17 cytokine production differentiates CD4+ T cells from lean, normoglycemic older and younger subjects, and mimics a diabetes-associated Th17 profile. T cells from older compared to younger subjects also had defects in autophagy and mitochondrial bioenergetics that associate with redox imbalance. Metformin ameliorated the Th17 inflammaging profile by increasing autophagy and improving mitochondrial bioenergetics. By contrast, autophagy-targeting siRNA disrupted redox balance in T cells from young subjects and activated the Th17 profile by activating the Th17 master regulator, STAT3, which in turn bound IL-17A and F promoters. Mitophagy-targeting siRNA failed to activate the Th17 profile. We conclude that metformin improves autophagy and mitochondrial function largely in parallel to ameliorate a newly defined inflammaging profile that echoes inflammation in diabetes
Conclusion: This is a cell culture study, not a human trial, so the usual caveat applies: it may fail in this respect for reasons as of yet unknown. However, as metformin has a well-known safety profile and is already approved by the FDA for other conditions, including diabetes itself, conducting a human trial to assess its effectiveness in stimulating autophagy is easier than testing a novel drug would be. We look forward to such a trial and hope for its success.
* Autophagy concerns cells own sel-reparing proxess, which can be part of self-care program in terms of starvation 14-18 hour each day.
Links:
https://www.hindawi.com/journals/jir/2016/8426874/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3348477/
https://www.cell.com/trends/endocrinology-metabolism/pdf/S1043-2760(16)30125-4.pdf
2021-05-07 More
https://rmdopen.bmj.com/content/7/1/e001470
Inflammaging as a link between autoimmunity and cardiovascular disease: the case of rheumatoid arthritis
Key messages
What is already known about this subject?
- ‘Inflammaging’ is characterised by the presence of persistent low-grade inflammation that develops with age and causes deficient tissue repair and degeneration, and it is reflected as an increase in the plasma levels of inflammatory cytokines and acute-phase reactants.
What does this study add?
- The low-grade, persistent pro-inflammatory milieu characteristic for the ageing process triggers morphological and functional changes, most prominently endothelial dysfunction, diffuse intimal–medial thickening and arterial stiffness.
How might this impact on clinical practice?
- The pattern of cytokine expression observed in autoimmune diseases, like rheumatoid arthritis, is similar to that seen in ‘inflammaging’ with similar consequences as increase vascular ageing induced by this chronic inflammation.
Abstract
Currently, traditional and non-traditional risk factors for cardiovascular disease have been established. The first group includes age, which constitutes one of the most important factors in the development of chronic diseases. The second group includes inflammation, the pathophysiology of which contributes to an accelerated process of vascular remodelling and atherogenesis in autoimmune diseases. Indeed, the term inflammaging has been used to refer to the inflammatory origin of ageing, explicitly due to the chronic inflammatory process associated with age (in healthy individuals). Taking this into account, it can be inferred that people with autoimmune diseases are likely to have an early acceleration of vascular ageing (vascular stiffness) as evidenced in the alteration of non-invasive cardiovascular tests such as pulse wave velocity. Thus, an association is created between autoimmunity and high morbidity and mortality rates caused by cardiovascular disease in this population group. The beneficial impact of the treatments for rheumatoid arthritis at the cardiovascular level has been reported, opening new opportunities for pharmacotherapy.
Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6146930/
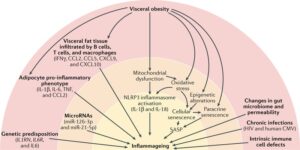
Fig. 1 |Potential causes of inflammageing.
Several genetic variants associated with high levels of inflammatory markers or increased response to inflammatory stimuli have been identified; the most relevant factors are indicated in parentheses. In central obesity, visceral fat tissue is infiltrated by T cells, macrophages, and monocytes. T cells secrete IFNγ, which stimulates the production of several chemokines by adipocytes, including C-C motif chemokine 2 (CCL2), CCL5, C-X-C motif chemokine 9 (CXCL9), and CXCL10, which further amplify tissue T cell infiltration. The number of B cells and macrophages in visceral adipose tissue from obese individuals is also increased and is correlated with BMI55. A specific subset of B cells expressing the tumour necrosis factor (TNF) superfamily ligand superfamily member 9 and producing TNF, IFNγ, and granzyme B accumulates in the abdominal cavity of older individuals56. Cytokines released by B cells contribute to the phenotypic change of adipocytes in the visceral cavity, causing them to release adipokines, other pro-inflammatory factors, and cell debris52. Activated monocytes that give rise to M1 and M2 macrophages produce even more inflammatory compounds57. Damaged mitochondria that cannot be repaired by repeated cycles of fission and fusion and are not recycled owing to defective autophagy release damage-associated molecular patterns (DAMPs) that trigger the NLRP3 inflammasome and lead to caspase 1-dependent production of IL-1β and IL-18. Oxidative stress is one of the possible triggers of cell senescence, which can be induced by several other stressors, including epigenetic alterations. Senescent cells, through the senescence-associated secretory phenotype (SASP), secrete large quantities of cytokines, chemokines, and other molecules, locally triggering more cell senescence (paracrine senescence) and contributing to inflammageing.
Studies have emphasized the role of age-related changes in the microbiome and increases in the gut mucosa permeability that lead to bacterial product release into the blood and stimulate an inflammatory response, in part through the NLRP3 inflammasome. In addition, part of inflammageing is probably caused by chronic infections (for example, human immunodeficiency virus (HIV) or human Cytomegalovirus (CMV) infection) and intrinsic defective mechanisms in immune cells that might involve metabolic stress as well as age-related changes in microRNA transcription. Of note, other important triggers of cell senescence, such as genomic instability, the activation of oncogenes, and the inhibition of tumour-suppressor genes, are not shown in the figure but might be part of the same mechanism.
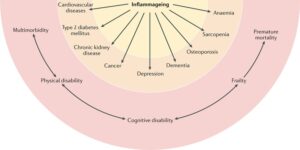
Inflammageing, defined as an age-related increase in the levels of pro-inflammatory markers in blood and tissues, is a strong risk factor for multiple diseases that are highly prevalent and frequent causes of disability in elderly individuals but are pathophysiologically uncorrelated. Mild chronic inflammation is generally considered to be a biomarker of accelerated biological ageing or one of the mechanisms by which the ageing process is associated with increased global susceptibility to all diseases. Cardiovascular diseases, chronic kidney disease, cancer, depression, dementia, osteoporosis, sarcopenia, and anaemia are shown in the figure as examples because extensive evidence indicates that inflammation contributes to the development of these diseases in old age, but the list is far from exhaustive3,138,139,142,143,196. Concordant with this view, elevated blood levels of pro-inflammatory markers (such as IL-6) are a powerful risk factor for multimorbidity (the number of coexisting diseases) and predict future rates of change in multimorbidity. Unsurprisingly, inflammageing is also a strong risk factor for typical geriatric conditions, such as physical and cognitive disability, frailty, and premature death. Although this effect is primarily mediated by multimorbidity, evidence also indicates that inflammation interferes with the maintenance and repair that constantly occur in all tissues, leading to accumulation of damage that contributes to frailty.
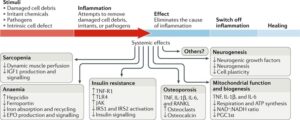
Fig. 3 |Inflammageing induces a catabolic state.
Inflammation causes pathological states linked with frailty, cardiovascular disease, and ageing. Sarcopenia: the induction of anabolic resistance in muscle inhibits the perfusion adjustment to anabolic stimuli as well as insulin-like growth factor (IGF1) production and signalling235–240. Anaemia: chronic elevation of IL-6 levels causes anaemia through the production of hepcidin, reduction of the transmembrane iron transporter ferroportin, and inhibition of iron absorption and recycling as well as interference with erythropoietin (EPO) production and signalling3,280. Insulin resistance: tumour necrosis factor receptor superfamily member 1A (TNF-R1) and Toll-like receptor 4 (TLR4) block insulin signalling through Janus kinase (JAK) activation, which causes serine phosphorylation of insulin receptor substrate 1 (IRS1) and IRS2, contributing to insulin resistance283. Osteoporosis: TNF, IL-1β, IL-6, and TNF ligand superfamily member 11 (RANKL) contribute to osteoporosis by stimulating osteoclast growth and activity and inhibiting the production of osteocalcin290,291. Mitochondria biogenesis: studies in vitro show that TNF, IL-1β, and IL-6 induce mitochondrial dysfunction with reduced ATP synthesis-driven respiration, a reduced NAD+:NADH ratio, and reduced mRNA levels of PPARGC1A (encoding peroxisome proliferator-activated receptor-γ co-activator 1α; PGC1α), suggesting impairment in mitochondrial biogenesis287. Neurogenesis: pro-inflammatory cytokines interfere with the biological activity of neuronal growth factors, such as brain-derived neurotrophic factor, thereby affecting neurogenesis and plasticity292. Accordingly, the addition of IFNα to human hippocampal progenitor cells reduces neurogenesis289. These are just few examples of how chronic inflammation promotes a catabolic state, suggesting a possible unifying hypothesis. During an acute bout of inflammation, induced for example by an infection, the surveillance of damage and continuous repair functions in multiple tissues are chronically inhibited, leading to accumulated damage in organelles and macromolecules. Over time, this damage accumulation across different tissues and organs could become so severe that it cannot be compensated for and causes irreversible frailty.
Conclusions
On the basis of the data and the hypotheses presented in this Review, modulating inflammageing is a promising strategy not only to prevent CVD but also to slow the decline of health that occurs with ageing. Modulating inflammation is likely to be most effective at the early stage of health decline, at a time when the compensatory capacity of the organism is not completely exhausted and might still counteract physiological and functional declines. New pharmacological treatments that selectively affect some of the signalling pathways that regulate inflammation are needed to balance the relationship between risks and benefits. Early treatments will require early diagnosis and availability of a signature biomarker profile that allows for a differential diagnosis between true inflammageing and chronic inflammation sustained by the persistence of an infectious or toxic cause. Ultimately, RCTs are needed to test the hypothesis that modulating inflammation prevents the development of CVD as well as multimorbidity, disability, and frailty.
Key points
- High levels of pro-inflammatory markers in the blood and other tissues are often detected in older individuals and predict the risk of cardiovascular diseases, frailty, multimorbidity, and decline of physical and cognitive function.
- In individuals with obesity, visceral fat produces pro-inflammatory and chemotactic compounds and is infiltrated by macrophages, lymphocytes, and senescent cells with a senescence-associated secretory phenotype that contributes to inflammageing.
- Mechanisms potentially underlying inflammageing include genomic instability, cell senescence, mitochondria dysfunction, microbiota composition changes, NLRP3 inflammasome activation, primary dysregulation of immune cells, and chronic infections.
- Clinical trials suggest that modulating inflammation prevents cardiovascular diseases, but studies to explore the effects on other chronic diseases, frailty, and disability are scarce and controversial.
- Inflammageing can complicate the clinical features of cardiovascular disease in older individuals by causing an energetic imbalance towards catabolism and interfering with homeostatic signalling, leading to frailty
Programmed PPAR-α downregulation induces inflammaging by suppressing fatty acid catabolism in monocytes
- https://www.sciencedirect.com/science/article/pii/S2589004221007343
Monocytes from aged individuals contained high levels of lipid droplets (LDs) - Downregulated PPAR-α is responsible for the aged monocytes profiles
- TNF-α might accelerate monocyte aging by downregulating PPAR-α expression
- PPAR-α activation in the elderly may also alleviate long-term inflammaging
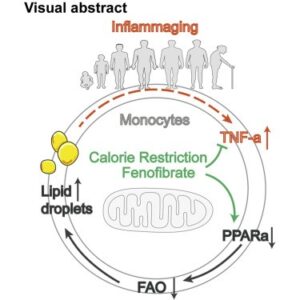
“Summary: Inflammaging is associated with an increased risk of chronic disease. Monocytes are the principal immune cells for the production of inflammatory cytokines and contribute to inflammaging in the elderly. However, the underlying mechanisms remain largely unknown. Here, we found that monocytes from aged individuals contained high levels of lipid droplets (LDs), and this increase was correlated with impaired fatty acid oxidation. Downregulated peroxisome proliferator-activated receptor (PPAR)-α may be responsible for the pro-inflammatory phenotype of monocytes in aged individuals, as it was positively correlated with LD accumulation and increasing TNF-α concentration.
Interestingly, interventions that result in PPAR-α upregulation, such as fenofibrate treatment, TNF-α neutralization, or calorie restriction, reversed the effect of aging on monocytes. Thus the downregulation of PPAR-α and LD levels in monocytes represents a novel biomarker for inflammaging. Furthermore, PPAR-α activation in the elderly may also alleviate long-term inflammaging, preventing the development of life-limiting chronic diseases.”
