Energy, Entropy and Quantum Tunneling of Protons … https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7927033/
BvS see below about specifically Neurodegeneration, e.g. ALS ”..
From www.Carism.se
- https://carism.se/2025-2/bo-von-scheeles-anamnes-dysfunctions-and-symptoms/ arthrosclerosis
- Tinnitus originating from within the auditory system https://carism.se/2021-2023/differential-diagnosis-tinnitus-syndrome-2023/ Otosclerosis, carotid atherosclerosis, Multiple sclerosis, https://treblehealth.com/otosclerosis-and-tinnitus/#:~:text=Otosclerosis%20is%20a%20disease%20of,issues%20with%20hearing%20%E2%80%93%20including%20tinnitus
- Also Otoskleroshttps://www.nhs.uk/conditions/otosclerosis/ + Otosclerosis is an abnormal bone growth in the middle ear, usually around the stapes (smallest hearing bone), that causes progressive conductive hearing loss, ringing (tinnitus), or dizziness (vertigo) by stiffening the bone and preventing sound vibrations from reaching the inner ear. It often starts in early adulthood, is more common in women, and has a genetic link, with pregnancy sometimes worsening it. Treatments include hearing aids, surgery (like stapedectomy), or cochlear implants
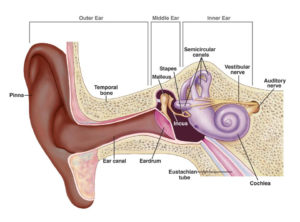
https://www.nidcd.nih.gov/health/otosclerosis - Tympanosclerosis vs Cholesteatoma: Tympanosclerosis is scarring/calcification (white plaques) on the eardrum (TM) or middle ear structures, often from prior infections or tubes, typically benign and only needing treatment if causing hearing loss. Cholesteatoma is an abnormal skin growth (keratin-filled sac) behind the eardrum that can invade bone, causing infection, foul discharge, and severe hearing loss, requiring surgery to prevent damage. Key differences: Tympanosclerosis is immobile scarring, while cholesteatoma is a growing mass, often behind the TM, and carries risk of bone destruction, making surgical removal crucial for cholesteatoma but not always for tympanosclerosis
Tympanosclerosis (Myringosclerosis)
What it is: White, chalky patches of calcium deposits and scarred collagen in the eardrum (myringosclerosis) or deeper in the middle ear (tympanosclerosis).
Appearance: Bright white plaques, often immobile on the TM.
Cause: Previous ear infections, ear tubes, or inflammation.
Symptoms: Usually none; can cause mild conductive hearing loss if extensive.
Treatment: Often none needed if asymptomatic; surgery if hearing loss is significant.
Cholesteatoma
What it is: A noncancerous skin cyst (keratinized epithelium) that grows, sheds skin, and can erode surrounding bone.
Appearance: Can appear as a white mass but is usually behind the eardrum and can cause erosions, not just calcification.
Cause: Chronic ear infections, eustachian tube dysfunction, or congenital.
Symptoms: Foul-smelling ear discharge, gradual hearing loss, sometimes dizziness or facial weakness.
Treatment: Surgical removal is usually necessary due to its destructive nature
Key Differentiators
Mobility: Tympanosclerosis is fixed to the eardrum; a cholesteatoma is a mass that can move or bulge.
Location: Tympanosclerosis is on or in the TM; cholesteatoma is behind the TM, in the middle ear space.
Risk: Tympanosclerosis is generally harmless scarring; cholesteatoma is an aggressive growth that destroys bone.
Treatment Goal: Tympanosclerosis treatment aims to improve hearing if affected; cholesteatoma treatment aims to stop bone destruction
- https://rarediseases.org/rare-diseases/tinnitus/“Muscular tinnitus can be caused by several degenerative diseases that affect the head and neck including amyotrophic lateral sclerosis or multiple sclerosis.
- (iv) 2017 Aggressive basal cell carcinoma, sclerosing, Glass type 3 https://carism.se/2024-2/2024-02-10-anamnes/
- a Pons dysfunction can also increase risks of ”small vessel disease” or ”amyloidosis” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9860402/ (The paradigm of amyloid precursor protein in amyotrophic lateral sclerosis: The potential role of the 682YENPTY687 motif) ”Neurodegenerative diseases are characterized by the progressive decline of neuronal function in several brain areas, and are always associated with cognitive, psychiatric, or motor deficits due to the atrophy of certain neuronal populations.
Most neurodegenerative diseases share common pathological mechanisms, such as neurotoxic protein misfolding, oxidative stress, and impairment of autophagy machinery.
Amyotrophic lateral sclerosis (ALS) is one of the most common adult-onset motor neuron disorders worldwide. It is clinically characterized by the selective and progressive loss of motor neurons in the motor cortex, brain stem, and spinal cord, ultimately leading to muscle atrophy and rapidly progressive paralysis. Multiple recent studies have indicated that the amyloid precursor protein (APP) and its proteolytic fragments are not only drivers of Alzheimer’s disease (AD) but also one of the earliest signatures in ALS, preceding or anticipating neuromuscular junction instability and denervation. Indeed, altered levels of APP peptides have been found in the brain, muscles, skin, and cerebrospinal fluid of ALS patients
https://carism.se/2024-2/microbleeds-in-middle-pons-ras-locus-coeruleus-arousal-no-habituation/
- ALS; Different kinds of ALS and more … https://carism.se/2024-2/2024-02-10-anamnes/als-olika-versioner/
Att kolla från www.boaim2.se
1. Possible explanation that healthy tissue becomes harder (sclerosis) https://www.boaim2.se/complex-diseases/possible-explanation-that-healthy-tissue-becomes-harder-sclerosis/
2. References: ”The autonomic nervous system (ANS) and the immune system are deeply interrelated. The ANS regulates both innate and adaptive immunity through the sympathetic and parasympathetic branches, and an imbalance in this system can determine an altered inflammatory response as typically observed in chronic conditions such as systemic autoimmune diseases. Rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis all show a dysfunction of the ANS that is mutually related to the increase in inflammation and cardiovascular risk. Moreover, an interaction between ANS and the gut microbiota has direct effects on inflammation homeostasis. Recently vagal stimulation techniques have emerged as an unprecedented possibility to reduce ANS dysfunction, especially in chronic diseases characterized by pain and a decreased quality of life as well as in chronic inflammation.” https://www.boaim2.se/clinical-focuses/the-inflammatory-reflex-and-how-transcutaneous-auricular-vagus-nerve-stimulation-may-activate-the-pathway/
Quantum tunneling
1. (also picture) https://www.boaim2.se/clinical-focuses/kvantpsykofysiologisk-beteendemedicin/
2. https://www.boaim2.se/innovativm2/tao-and-quantum-and-more/tao-quantum-psychophysiological-behavioral-medicine-projects/why-combine-quantum-biology-medicine-with-tao-and-chi-kung/
- Rethinking Disease: How the Quantum Biology of Ferritin Could Change the Game | by Chris Rourk https://www.boaim2.se/qm/quantum-medicine-2024/neurodegenerative-diseases-arise-from-oxidative-damage-to-electron-tunneling-proteins-in-mitochondria/rethinking-disease-how-the-quantum-biology-of-ferritin-could-change-the-game-by-chris-rourk/
- Even more … to elaborate
InflammAging and sclerosis/artritis similaries and differences
2024-08-14
Molecular Basis of Accelerated Aging with Immune Dysfunction-Mediated Inflammation (Inflamm-Aging) in Patients with Systemic Sclerosis
https://www.mdpi.com/2073-4409/10/12/3402
“Systemic sclerosis (SSc) is a chronic connective tissue disorder characterized by immune dysregulation, chronic inflammation, vascular endothelial cell dysfunction, and progressive tissue fibrosis of the skin and internal organs. Moreover, increased cancer incidence and accelerated aging are also found. The increased cancer incidence is believed to be a result of chromosome instability. Accelerated cellular senescence has been confirmed by the shortening of telomere length due to increased DNA breakage, abnormal DNA repair response, and telomerase deficiency mediated by enhanced oxidative/nitrative stresses. The immune dysfunctions of SSc patients are manifested by excessive production of proinflammatory cytokines IL-1, IL-6, IL-17, IFN-α, and TNF-α, which can elicit potent tissue inflammation followed by tissue fibrosis. Furthermore, a number of autoantibodies including anti-topoisomerase 1 (anti-TOPO-1), anti-centromere (ACA or anti-CENP-B), anti-RNA polymerase enzyme (anti-RNAP III), anti-ribonuclear proteins (anti-U1, U2, and U11/U12 RNP), anti-nucleolar antigens (anti-Th/T0, anti-NOR90, anti-Ku, anti-RuvBL1/2, and anti-PM/Scl), and anti-telomere-associated proteins were also found. Based on these data, inflamm-aging caused by immune dysfunction-mediated inflammation exists in patients with SSc. Hence, increased cellular senescence is elicited by the interactions among excessive oxidative stress, pro-inflammatory cytokines, and autoantibodies. In the present review, we will discuss in detail the molecular basis of chromosome instability, increased oxidative stress, and functional adaptation by deranged immunome, which are related to inflamm-aging in patients with SSc”.
What kinds of all kinds of sclerosis are existing? https://www.mssociety.org.uk/about-ms/what-is-ms
“’Sclerosis’ means scarring and refers to the scars (also called lesions) that MS causes in your brain or spinal cord. These show up in magnetic resonance imaging (MRI) scans. It’s ’multiple’ sclerosis because the lesions happen in more than one place.”
BvS – A problem is that it is written very MUCH about MS but not of ALS and its shared dysfunction, which can be read between the text lines. What we need to focus much on is how biologically sclerosis any where develops while health tissue is slowly (?) gradually change into dysfunctional, probably partly related to dysfunctions of mitochondria (local or general) as well as differential diagnoses hypotheses, e.g. see below
Which Diseases Are Similar to MS? https://www.healthcentral.com/condition/multiple-sclerosis/diseases-similar-to-ms
2022-03-08
Inflammaging: Age and Systemic, Cellular, and Nuclear Inflammatory Biology in Older Adults https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6777092/ “STAT signal transducer and activator of transcription … Within a community-dwelling sample of older adults, older age is associated with increases in STAT activation, along with increases of systemic inflammatory cytokines. In older adults, heterogeneity in age-related increases in inflammatory disease risk may be related to individual variability in inflammation”
Age-related cerebral small vessel disease and Inflammaging https://www.nature.com/articles/s41419-020-03137-x
“Circulating biomarkers of CSVD (cerebral small vessel diseases) inflammation were classified as markers of systemic inflammation and markers of vascular inflammation/endothelial dysfunction6. Besides, four core MRI features have been identified as imaging markers of CSVD, namely white matter hyperintensities (WMH), lacunae, cerebral microhemorrhage (CMB), and perivascular space enlargement (EPVS)56.” … The regional analysis showed blood markers of vascular inflammation are often associated with deep perforating arteriopathy (DPA), while blood markers of systemic inflammation appear to be associated with cerebral amyloid angiopathy (CAA). Here, we discuss recent findings in the pathophysiology of inflammaging and their effects on the development of age-related CSVD. Furthermore, we speculate the inflammaging as a potential target for future therapeutic interventions to delay or prevent the progression of the age-related CSVD.
Fig. 1: The deep interactions between aging, inflammaging, and age-related CSVD.
As aging, several cellular and molecular mechanisms lead to chronic inappropriate activation of the immune system. This complex interaction between genetic susceptibility and risk stimuli (both exogenous and endogenous) contributes to the continuous activation of a limited range of confounding sensors which triggers inflammaging (upper part of the box). The resulting synthesis and release of different inflammatory mediators are related to the common pathophysiological mechanisms of age-related diseases. For age-related CSVD, regional analyses showed that blood markers of vascular inflammation were associated with deep perforating arteriopathy (DPA), while blood markers of systemic inflammation were associated with cerebral amyloid angiopathy (CAA), both of which were closely related to the critical pathophysiological mechanisms of blood-brain barrier leakage and endothelial dysfunction (lower part of the box).”
InflammAging & Tinnitus
Neuroinflammation mediates noise-induced synaptic imbalance and tinnitus in rodent models https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.3000307 “…This excitatory-to-inhibitory synaptic imbalance was completely prevented by pharmacological blockade of TNF-α expression. These results implicate neuroinflammation as a therapeutic target for treating tinnitus and other hearing loss–related disorders.”
Chronic Inflammation – Inflammaging – in the Ageing Cochlea: A Novel Target for Future Presbycusis Therapy https://www.researchgate.net/publication/320354252_Chronic_Inflammation_-_Inflammaging_-_in_the_Ageing_Cochlea_A_Novel_Target_for_Future_Presbycusis_Therapy
Chronic myelitis: Inflammaging
Emerging roles of frailty and inflammaging in risk assessment of age-related chronic diseases in older adults: The intersection between aging biology and personalized medicine – https://www.researchgate.net/publication/273490290_Emerging_roles_of_frailty_and_inflammaging_in_risk_assessment_of_age-related_chronic_diseases_in_older_adults_The_intersection_between_aging_biology_and_personalized_medicine
An Update on InflamAging: Mechanisms, Prevention, and Treatment
Inflammation in CNS neurodegenerative diseases – https://onlinelibrary.wiley.com/doi/full/10.1111/imm.12922
Inflammation and its resolution and the musculoskeletal system – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5541893/
Anemia at older age: etiologies, clinical implications, and management – https://www.sciencedirect.com/science/article/pii/S0006497120325088
Aging and multiple sclerosis – https://journals.sagepub.com/doi/full/10.1177/1352458516634871 “In this brief literature review, we consider how advancing age influences clinical disease course and pathological and immunological processes implicated in MS. Furthermore, we discuss how several MS symptoms interact with the aging process and advise the clinician how clinical findings may indicate normal aging, relentless MS disease progression, a new disease common in aging, or some combination of these. The optimal differential diagnosis will lead the clinician to the most appropriate therapeutic approach
